Laboratory & Professor in the College of Chemistry
Researches in the College of Chemistry
Laboratories
In the fourth year, students belong to one of the following laboratories as they conduct their graduation research. If you wish to conduct more specialized research, you can go on to a graduate school after graduation. There are other laboratories in the graduate school. For more information about entrance examinations and other details, please see the website of the Master’s/Doctoral Program in Chemistry, Graduate School of University of Tsukuba.
-
Inorganic Reaction Chemistry
Synthesis of transition-metal complexes and their reactivity in various redox and catalytic reactions; supramolecular redox chemistry of non-planar and fused porphyrins.
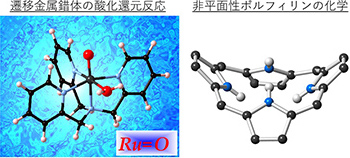
-
Synthetic Inorganic Chemistry
Creation of multi-nuclear metal clusters with controlled structures and electronic states; chemistry of functional metal complexes with controlled electronic states and structures.
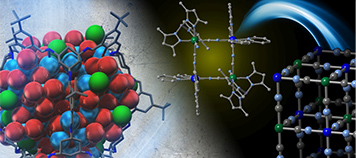
-
Analytical Chemistry
Studies on chemical processes at microdroplet/solution and microparticle/solution interfaces using electrochemical and spectroscopic techniques.
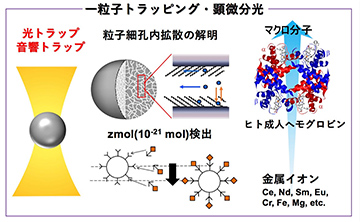
-
Radiochemistry
Studies of the physical and chemical properties, behaviour and environmental origins of natural and anthropogenic radionuclides. Use of radionuclides to assess environmental dynamics and solve environmental problems. Research on artificial nuclear transmutation, measurements of nuclear reaction cross sections and nuclear reaction mechanisms.

-
Molecular Condensed Matter
StaffsStructure and property of soft molecular systems, and dynamics and phase transitions in them.
-
Spectroscopic Physical Chemistry
Studies on interfaces and condensed phases by linear and nonlinear molecular spectroscopy; Studies on photofunctions and photochemical properties of newly fabricated molecular assemblies and inorganic particles in mesoscopic scale.
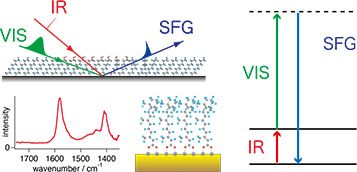
-
Atmospheric Physical Chemistry
StaffsAtmospheric multiphase chemistry, biosurface chemistry, molecular-level inhomogeneity on/in liquid phases, interface chemistry and physics, and development of novel experimental methods.
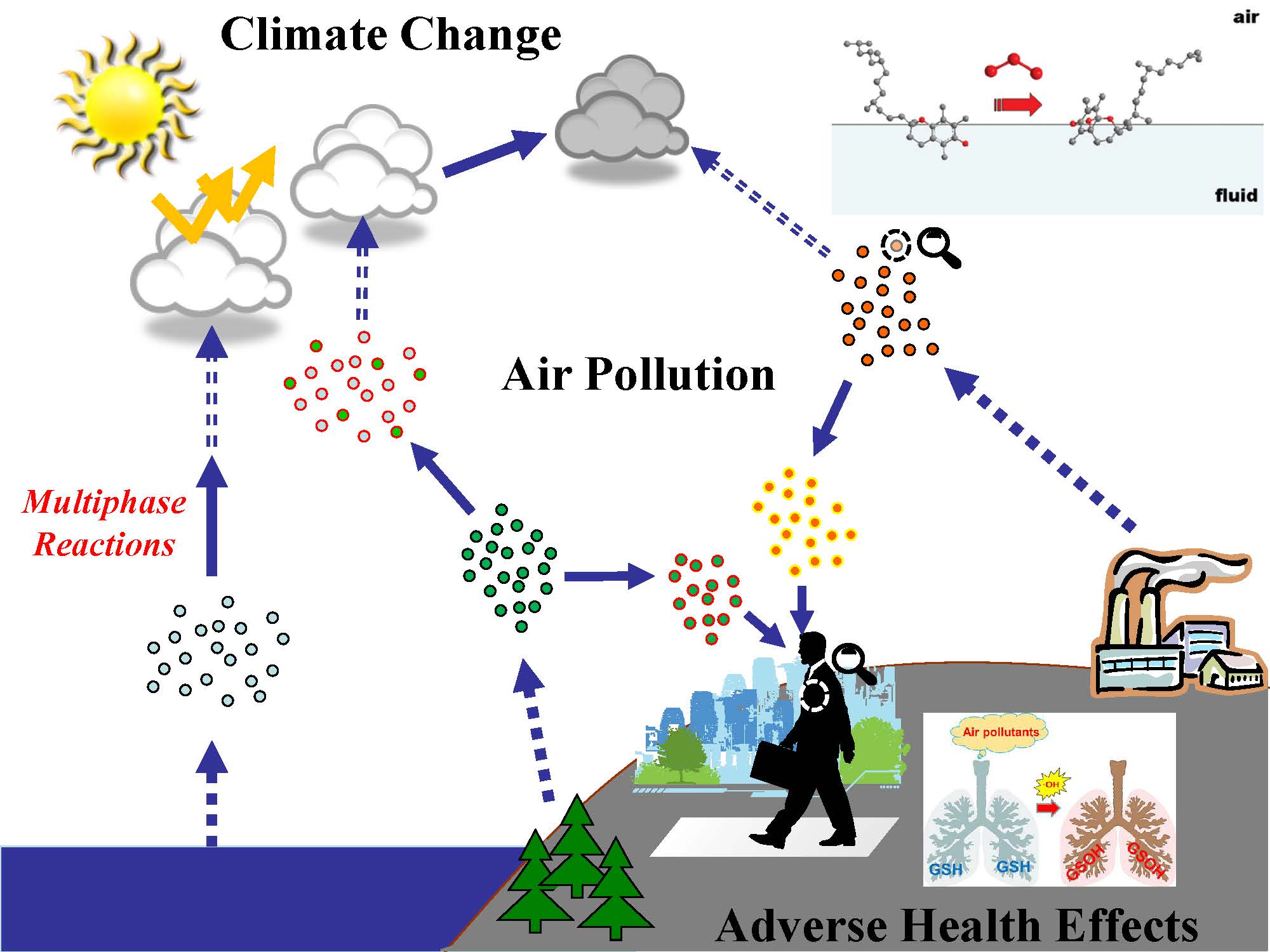
-
Chemical Computation & Information
Elucidation of molecular mechanism and molecular design of complex molecular systems based on computational chemistry and information science. Computational research including quantum chemical calculations and molecular dynamics simulations: development for determining the molecular properties by solvation model and density functional theory.
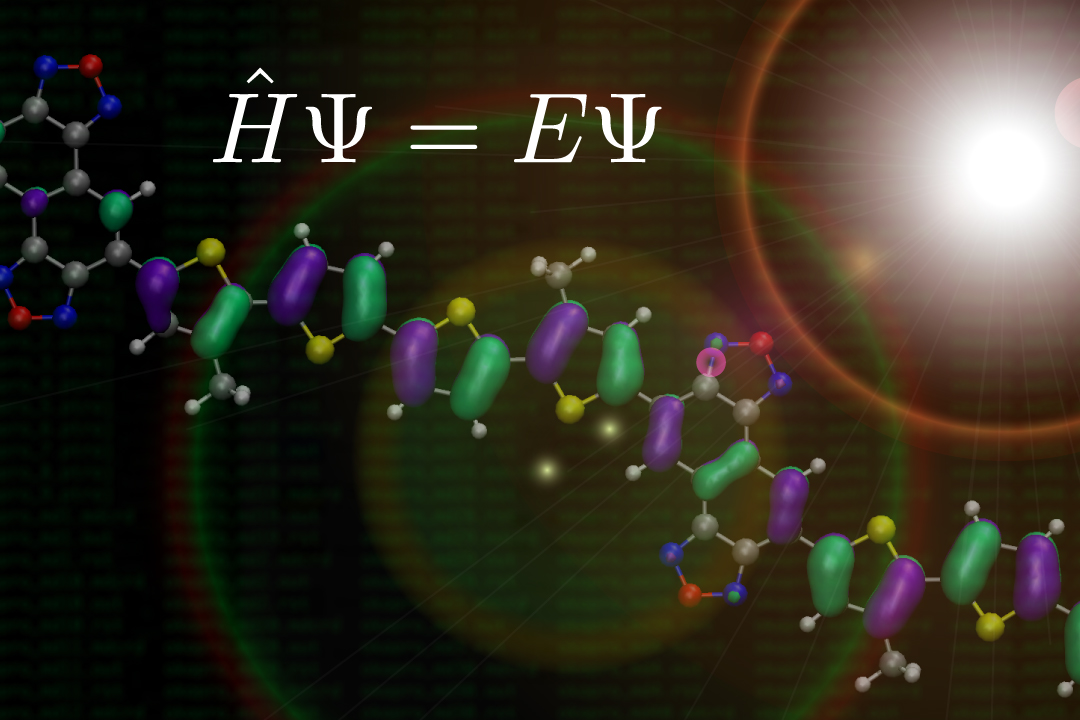
-
Organoelement Chemistry
Low-coordination and multiple-bonded compounds of heavier group 14 elements and organoelement chemistry of Group 13-14.
-
Bioorganic Chemistry
StaffsIsolation, structural elucidation, synthesis, and bioorganic studies of bioactive natural products. Design, synthesis and biological evaluation of novel biologically active molecules.

-
Supramolecular Chemistry
StaffsPrecise construction of functional molecules based on supramolecular chemistry, and exploration of their properties such as molecular recognition and selective reaction. Studies on supramolecular metal complexes utilizing organic ligands and metal ions.

-
Medicinal Chemistry
StaffsDrug discovery for controlling sleep/wakefulness, Synthesis of bioactive molecules, Studies of chemoselective reaction useful for drug discovery
-
Structural Biology and Chemistry
Study of proteins in soft-tissue sarcoma, chromatin remodeling factors and a photosensing flavoprotein. Structural biology and chemistry using single-particle electron microscopy and its development.




